ARCHIVED - Government of Canada Response to the Report of the Expert Review Panel on Medical Isotope Production
Information Archived on the Web
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats. Please "contact us" to request a format other than those available.
PDF Version (485 Kb)
Introduction
Recent supply disruptions have highlighted the fragility of the supply chain that delivers essential medical isotopes to patients globally. A new and more reliable way of supplying isotopes to Canadians needs to be found. That is why the Government of Canada established the Expert Review Panel on Medical Isotope Production (the Panel) in June 2009.
The Government recognizes the relatively long lead times associated with the development of any new source of medical isotopes. To ensure that appropriate action is taken now for the long term, the Government tasked the Panel with reporting to the Minister of Natural Resources on its assessment of the most viable options for securing supplies of technetium-99m (Tc-99m) for the Canadian health care system over the medium and long term and the actions that may be required by governments and others to facilitate realization of these options.
The Panel reported to the Minister of Natural Resources on November 30, 2009. Since then, the Government has been carefully considering the recommendations of the Panel within the context of the broader nuclear and health care landscape.
What follows is the Government’s response to the Panel’s thoughtful, comprehensive and insightful report, including actions that are planned based on its recommendations.
Background
Tc-99m, which is obtained from the decay of its parent isotope molybdenum-99 (Mo-99), is the most widely used medical isotope for medical imaging and accounts for approximately 80 percent of nuclear medicine diagnostic procedures. Tc-99m performs a critical role in the diagnosis of heart disease and is also used in cancer diagnosis through bone and organ scans.
Radio-isotopes (isotopes) naturally decay into more stable substances over time, some more quickly than others (the decay is what allows the diagnostic image to be captured). The half-life of an isotope is the time required for a quantity of radioactive material to decay to half of its initial amount. Given the relatively short half-life of Mo-99 (66 hours) and the even shorter half-life of Tc-99m (six hours), it cannot be stockpiled for later use. To ensure continuous availability, Mo-99 must be produced frequently, which adds to the complexity of the supply chain.
Historically, virtually all of the commercial supplies of Mo-99 have been produced via fission in nuclear reactors. Furthermore, since the United States (U.S.) does not produce Mo-99, the North American market has traditionally been heavily dependent on Atomic Energy of Canada Limited’s (AECL) National Research Universal (NRU) reactor for the production of Mo-99, and subsequently for its supplies of Tc-99m.
In Canada, home to the NRU reactor located in Chalk River, Ontario, the medical isotope supply chain has been complex and has involved a combination of both public- and private-sector organizations. The supply chain typically functions as follows. Highly enriched uranium (HEU) targets (obtained from the U.S.) are irradiated in the publicly-owned NRU reactor and processed for Mo-99 extraction. The raw Mo-99 supplied from the NRU is then sent to MDS Nordion, a private-sector company in Kanata, Ontario, for further processing and purification. Once MDS Nordion has purified the Mo-99, it is then shipped to Tc-99m generator manufacturers in other countries. Most of the product is shipped to U.S. private-sector Tc-99m companies that manufacture the generators and then sell to Canada through their Canadian subsidiaries.
Although Canada is the largest Mo-99 producer in the world when the NRU is operational, the supply chain is international and involves a number of other private sector players. A failure at any point in the supply chain can impact security of supply.
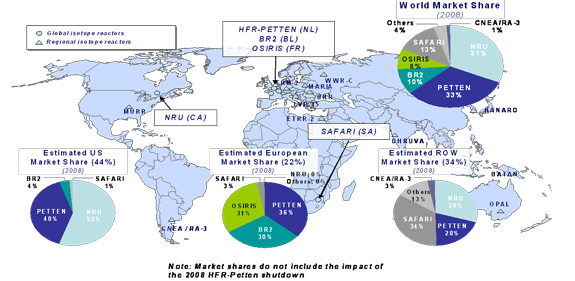
Moreover, nearly all of the world’s supply of Mo-99/Tc-99m is met by five aging nuclear research reactors, with the NRU reactor providing approximately 30 to 40 percent of that supply. The other major isotope-producing research reactors are located in the Netherlands, South Africa, Belgium and France. However, some small but important additions to the supply chain are slowly emerging and are discussed below.
Recent outages of a number of these isotope-producing research reactors have highlighted the vulnerabilities in the current global supply chain, which are likely to remain for some time.
Security of supply is not a function of reactor reliability alone. It is also a function of processing capacity and availability, geographic alignment between processors and reactors, transport, handling and efficient use of the product. Historically, supply chains have been linear and somewhat independent, giving rise to the risk of complete breakdown if there is failure in one link of the supply chain (i.e., the single-point-of-failure risk). It should be noted that the relations among all players in the supply chain have been evolving since the outage of the NRU, and many of the issues noted above are being examined and improved upon.
Additionally, Canada has taken a leadership role internationally, working with its international partners to draw attention to the fragility of global medical isotopes supply and to mobilize international approaches to the problem. At Canada’s instigation, a High Level Group (HLG) on the Security of Supply of Medical Radioisotopes was created under the auspices of the Nuclear Energy Agency of the Organization for Economic Cooperation and Development to bring together key international participants in the supply and demand chain.
Within the HLG, a consensus was reached on the need to improve the coordination of reactor schedules, increase transparency, improve the efficiency of the distribution system, and provide timely notification of available supplies to the medical community.
Significant efforts have been made by suppliers and the international community to limit the public's exposure to upcoming shortages in 2010. For example, Belgium has added a cycle to its reactor schedule, South Africa will continue to operate at elevated levels, France has agreed to delay a scheduled outage, and Australia is working diligently to get new product into the international market in 2010. Further, on February 17, 2010, there was an announcement that the MARIA reactor in Poland expects to be supplying 7 to 10 percent of the world market for the first time by late March 2010.
Additional efforts to respond to the recent supply disruptions have been focused on mitigation strategies and communications, with regular updates provided to the medical community to allow it to plan and adjust as necessary. With strong engagement by Health Canada, the medical community has adapted its practices, utilizing isotopes more efficiently and finding alternatives for a number of procedures.
The Report of the Expert Review Panel on Medical Isotope Production
The Panel found that a sustainable supply of Tc-99m would have the following characteristics:
- “be viable for the foreseeable future, likely for at least 15 to 20 years, and may include options that begin producing in the short to medium timeframe but that promise to remain viable;
- comprise options that could each meet a meaningful portion of the Canadian demand, but that would not necessarily be exclusively Canadian-based and may or may not serve the U.S. or other markets;
- have a sound business model that may or may not include government involvement; and
- be free of highly enriched (weapons-grade) uranium because of Canadian and global commitment to non-proliferation.” (Panel, 2009)
The Panel also found that a secure supply of Tc-99m would:
- “improve redundancy at all points in the supply chain to avoid the single point of failure risk associated with a linear supply chain;
- use diverse technologies to hedge against a failure that could arise if all suppliers used the same technology;
- collocate irradiation and processing facilities to minimize decay losses and avoid shipping losses and risks; and
- ensure sufficient capacity to accommodate short-term outages of some sources.” (Panel, 2009)
Establishing these parameters for sustainable and secure supply helped to frame how the Panel assessed the likelihood of various technology options contributing to a stable isotope supply in the long term. These parameters will also help the Government of Canada frame the issue.
Building on this framework, the Panel provided the following general recommendations:
- strive for diversity and redundancy throughout the supply chain;
- leverage multi-use infrastructure;
- continue with international coordination and seek processing standardization within North America; and,
- recognize that HEU options are viable only in the short to medium term.
The Government of Canada finds the general recommendations to be useful in evaluating options under consideration. Like the Panel, the Government believes that a reliable, resilient global supply chain cannot be overly dependent on any one source, Canadian or otherwise. The global community must continue to work together to make every effort to improve the resiliency of the global supply.
The Panel’s recommendation regarding HEU is in line with Canada’s commitment to non-proliferation. Also, the Government recognizes the potential impacts of pending U.S. legislation that would see the eventual elimination of HEU exports from the U.S., thereby cutting off supplies of the raw material needed for Canadian and global production of Tc-99m by traditional methods.
Overview of the Government’s Priorities for Secure Isotope Supply
Despite the efforts being made in the short term to secure isotope supply and to support health care system mitigation measures, the Government of Canada recognizes that these are not all sustainable. Investment is needed now for work that will increase the security of isotope supply in the medium to long term. Based on the Panel’s advice, the Government is taking action to ensure that continued and alternative supply options are available. The NRU’s licence will expire in 2011, and there are no concrete plans internationally that would ensure replacement of the NRU reactor’s production in the short to medium term. This is why investments are being made to relicense the NRU to 2016. However, it is not the intention to have the NRU produce isotopes beyond 2016. Investment in non-reactor-based production is intended to support development of non-federal supply options that will serve well beyond 2016.
The Government of Canada has asked AECL to make return to service of the NRU its top priority, and the federal budget has provided money for AECL’s ongoing operations including relicensing activities. Moreover, the Government has developed an action plan to increase the security of medical isotope supply for Canadians in the longer term based on i) encouraging new long-term supply of medical isotopes by investing in research and development to prove new technologies with commercial potential, ii) supporting resiliency and effective health system management by optimizing the use of available supplies and alternatives, where appropriate, and iii) continuing to work with the international community to coordinate outages and production of medical isotopes.
The Government of Canada is looking to transform the way Canada produces medical isotopes, and in particular Tc-99m, so that Canadian production is on a sound commercial footing without government support; production is scaled to the needs of Canadians; it is sustainable in terms of environmental impacts, health, safety and security; and Canada remains a global technological leader. We believe that this transformation will best serve the needs of Canadians for a secure supply of medical isotopes in the medium and longer term. Canada’s NRU reactor has satisfied a significant portion of world demand for Mo-99; by producing at this scale, Canadians have been left to shoulder a disproportionate amount of the nuclear waste burden associated with reactor-based isotope production. This includes the significant costs associated with long-term management of the waste. The Government favours a new paradigm in which Canadians benefit from Canadian-based isotope production, supplemented if necessary from the world market, and supply is sustainable because of reduced waste and improved economics.
Canada has been at the forefront of medical isotope technology since the first cobalt-60 teletherapy units were used in Saskatchewan and Ontario in 1951. Canada's technological leadership can be maintained and enhanced as we move to a new and more sustainable model of isotope production based on Canadian needs. The Expert Review Panel has pointed to promising new technologies for the future production of Tc-99m. These technologies are being used today for other purposes. The cyclotron and accelerator technologies advocated by the Panel are ones in which Canada is already an established leader, including for the production of PET isotopes and for scientific research. Investing in research, development and demonstration (RD&D) to test the viability of these technologies for commercial production of Tc-99m can only enhance Canada's technological leadership, as well as enable a focus on meeting Canadian needs more sustainably.
By developing supply options that are scaled to Canada’s needs, Canada will be creating new intellectual property and new businesses. Canada will be putting in place options that could benefit smaller international markets that have not been able to justify the “big investments” for “big production” that are required for reactor-based production. Non-reactor-based production would potentially allow smaller nations the opportunity to consider domestic production of Tc-99m for the first time, and would establish Canada as a technology leader in new and emerging technologies.
From a regulatory point of view, the Government is preparing the path forward to enable new sources of medical isotopes to be licensed in a timely manner while ensuring the highest standards of safety and security. The regulatory departments and agencies will work proactively to ensure efficient and effective regulatory processes. In particular, the Canadian Nuclear Safety Commission (CNSC) is planning to work with proponents of these alternative technologies to ensure regulatory requirements are clearly understood well in advance of the need to seek regulatory approvals.
Detailed responses to the Panel’s technology-specific recommendations are given below.
Specific Recommendations and the Government’s Responses
Expert Panel Specific Recommendation 1: Make policy decisions on the requirement for a new research reactor.
Panel Recommendation
We recommend that the government expeditiously engage in the replacement of the NRU reactor as we believe a multi-purpose research reactor represents the best primary option to create a sustainable source of Mo-99, recognizing that the reactor’s other missions would also play a role in justifying the costs. With the National Research Universal (NRU) reactor approaching the end of its life cycle, a decision on a new research reactor is needed quickly to minimize any gap between the start-up of a new reactor and the permanent shutdown of the NRU. If the decision is to not build a new research reactor, the issue of securing supply of Tc-99m will have to be revisited in light of how cyclotron/accelerator options are advancing, and what new foreign sources of isotopes have materialized.
Government of Canada Response
The Panel expressed support for a new reactor, while emphasizing that what is most important in the short term is making a quick decision on this issue so that the market can adjust appropriately. The Panel’s support for a new multi-purpose research reactor was based on a belief that this was the least technologically risky approach. However, the Panel recognized that a new research reactor was a very expensive piece of infrastructure that could not be justified based on isotope production alone.
From a purely isotope perspective, outside the considerations of the other missions of a research reactor, the Government finds that the very high costs and very long lead times make this a less attractive option than others. Based on the experience of other countries, it would likely take a decade or more to bring a new research reactor on stream. Also, the significant fixed costs and production capacity would be disproportionate to Canada’s isotope needs and could not be recouped from the market. Waste liabilities associated with long-term reactor-based isotope production would be significant and again difficult to fully recover. Moreover, while production of medical isotopes using HEU targets is well established, there remain technical challenges associated with yields using low enriched uranium (LEU) targets, and the volume of waste would increase significantly.
A research reactor is only one piece of the linear supply chain that exists today. Replacing one piece of a linear supply chain, such as simply replacing the NRU with another reactor, would do little to develop the diversity and redundancy that the Panel believed were critical for ensuring security of supply. The lesson learned is that more should be done to create cross-linked and distributed supply chains that are not as vulnerable to single-point failures. An announcement that a new research reactor would be built in Canada to produce medical isotopes would discourage investment in alternative sources of supply, both in Canada and in other countries. The supply chain would continue to remain vulnerable to the single-point-of-failure problem that exists today, and generators would likely be manufactured outside of Canada. Also, purchasing “off-the-shelf” reactor technology from a foreign vendor would do little to maintain Canada as a leader in isotope technology.
A research reactor serves many missions. The need for a new reactor for these other purposes would need to be based on a thorough assessment of the missions, including neutron scattering and R&D for the nuclear industry, and consideration of the appropriate sharing of costs among the many users and beneficiaries of such a facility. This assessment is outside the scope of this response.
Expert Panel Specific Recommendation 2: Support an R&D program for cyclotron-based Tc-99m production.
Panel Recommendation
We recommend that the cyclotron option for direct production of Tc-99m, which has many attractive features, be explored further. Although this option requires significant R&D, the infrastructure and know-how to undertake that work is readily available in Canada, so costs associated with the R&D remain relatively low. Assuming technical viability, the infrastructure necessary to demonstrate this approach in selected centres across Canada is already in place. Indeed, Canada has an opportunity to be a leader in this area and strengthen its existing related businesses.
Government of Canada Response
The cyclotron option holds considerable promise for significant advantages over other alternatives. Several cyclotron facilities are already in place across Canada. These cyclotrons are already producing and distributing other medical isotopes, some of which have even shorter half-lives than Tc-99m.
This option would introduce a more distributed network of supply hubs that would eliminate the single point of failure problem that makes today’s supply chain so vulnerable.
An important consideration from cost-savings and environmental perspectives is that this option would largely avoid nuclear waste issues.
Investment in the development of this option is low risk because even if the Tc-99m production technology does not prove successful, or if demand for Tc-99m diminishes over time, the facilities would continue to be useful for other missions, namely, producing positron emission tomography (PET) medical isotopes.
Another feature of the cyclotron approach is that it has the prospect of being economically viable without the need for ongoing government support. The Government of Canada sees this as a necessary evolution as government support in several countries have impeded healthy market development and created a vulnerable supply chain based on a limited number of producers using aged facilities.
It is important to recognize that significant research and development is needed to fully prove out this method and to demonstrate that high-quality Tc-99m can be produced at reasonable cost using cyclotrons. At its current stage of development, there are uncertainties around the quantity and purity of the product that can be produced, and the cost and availability of the required raw material.
The Government plans quick action to provide $35 million for research, development and demonstration of non-reactor-based technologies for the production of Tc-99m, including cyclotrons. The proposed program would fund a range of activities from applied research up to and including demonstration activities to expedite the progression of the cyclotron and linear accelerator technologies from the lab to the market. The linear accelerator option is discussed further below in the section entitled Other Considerations.
A funding program is being developed and the Government expects to issue a competitive request for proposals in the spring of 2010.
Expert Panel Specific Recommendation 3: Achieve better use of Tc-99m supply through advanced medical imaging technologies
Panel Recommendation
We recommend deployment of newer single photon emission computed tomography (SPECT) technologies (software and hardware), as well as investment in positron emission tomography (PET) technology, to reduce demand for Tc‑99m now and over the longer term, which would reduce the impact of future shortages of reactor-produced isotopes.
Government of Canada Response
The Government of Canada recognizes the importance of increased efficiency in the use of Tc-99m as well as diversification of imaging technologies and isotopes, as a means of reducing reliance on Tc-99m. The investments outlined below, as announced in Budget 2010, will support the medical community and governments in continuing to provide effective and appropriate medical imaging services, as approaches to medical isotope supply and other factors within the broader health care system evolve over time.
In line with the federal role in leading and fostering research and innovation, the Government of Canada will support the further diversification of advanced medical imaging technologies, and encourage the development of new or alternative isotopes by creating a Medical Imaging Clinical Trials Network (CTN). An investment of $5 million/year over two years, led by the Canadian Institutes of Health Research (CIHR), will help establish the Medical Imaging Clinical Trials Network as the first Network within CIHR’s Strategy for Patient-Oriented Research. It will be informed by excellent research funded to date on medical imaging, including the CIHR/Natural Science and Engineering Research Council of Canada (NSERC) Medical Imaging Workshop Report written October 2009.
Establishing a multidisciplinary research Medical Imaging Clinical Trials Network will facilitate the rapid translation of advances in medical imaging into applications useful to health professionals in patient diagnosis and treatment, and the life sciences industrial sector for the benefit of the economy. Compared with funding individual research initiatives, networks offer the added benefit of: creating a critical mass of international scientific expertise; linking relevant support and other centres of researchers; coordinating overall research activities of the network; linking to community-based care programs; and facilitating continuity in research.
The Government of Canada will also support its health care system partners in optimizing the use of available Tc-99m and alternative imaging technologies where appropriate. To this end, the Government of Canada will invest $3 million over two years for the development of tools, protocols and standards for improved efficiency and effectiveness in health system management of medical imaging. As noted by the Panel, the health care community has worked hard to put mitigation strategies in place during recent periods of supply disruption. While not all of these strategies are sustainable or desirable over the longer term, there have been real efficiency gains and clinical learnings that should not be lost.
This initiative will support a leading national organization to engage experts, provider communities, provinces and territories, health technology and health service organizations to build on the networks, guidelines, and practices that have been established, synthesize various lines of evidence, and draw on innovations, research and lessons learned. The materials produced, which would harness and further develop learnings to date, will inform decision making at all levels, including decisions about the efficient and effective imaging technologies now and over the longer term.
Other Considerations
Expert Panel Consideration 1: Linear accelerator options
The two linear accelerator options have limited prospects for multi-purpose use, require significant R&D, and may not have significant cost advantages over reactor technologies. Nonetheless, a modest R&D investment could be considered as a hedge against the risk of failure of other options. Of the two linear accelerator options, we prefer the technology based on Mo-100 transmutation since the projected economics appear better, and it largely avoids nuclear waste management issues.
Government of Canada Response
The Panel found that the Mo-100 transmutation technology was the more attractive of the two linear accelerator options. Like the cyclotron technology, the Mo-100 transmutation technology is non-reactor-based, avoiding many of the challenges associated with constructing and operating reactors. Especially significant for the Government is the avoidance of significant nuclear waste management issues, thereby reducing cost and minimizing environmental impact.
However, the Government recognizes that there are important differences between these two methods:
- The cyclotron option would produce Tc-99m directly, without first generating Mo-99. Because the half-life of Tc-99m is short (six hours), producing Tc-99m directly means that processing, distribution and storage times must be very short. This may limit the range of distribution for this product, making the cyclotron option a more localized and regional solution.
- The Mo-100 transmutation option would be based on one or two facilities in Canada and may require a new type of generator technology; whereas the cyclotron option would be based on a distributed network of regional facilities.
- Relying on one or two accelerators may result in the same supply chain vulnerabilities that exist today, based on potential for single point of failure.
Overall, the linear accelerator and cyclotron options are at a similar stage of development, and questions remain regarding economic projections, technical challenges, ease of regulatory approval and market acceptance.
Despite the uncertainties, the Government notes that the Panel found that the cyclotron and the Mo-100 transmutation options are both potentially attractive because they have the potential to be economically viable without the need for ongoing government support.
There is merit in having the two technologies compete to stimulate the best ideas and to stimulate the best commercial prospects. Also, given that they are at a similar stage in terms of development, the Government finds it prudent to invest in both technologies until the viability of each is better understood. Ultimately, one or both of these non-reactor technologies may find a place in the market.
As mentioned earlier, a $35 million funding program is being developed, which would invest in the research, development and demonstration (RD&D) of non-reactor-based technologies for the production of Tc-99m. This program would consider proposals for RD&D activities for the cyclotron or Mo-100 transmutation options.
Expert Panel Consideration 2: the MAPLEs
Panel Observations
Cost and timeline estimates associated with the commissioning and licensing of the MAPLE reactors varied widely. Although it may be possible to bring them into operation, the business case is such that even if the facilities could be licensed immediately at no cost, the ongoing revenues from isotope sales would be insufficient to cover the ongoing operating expenses, particularly with the anticipated reduced throughput from future conversion to LEU targets. A dedicated isotope facility based on a private sector cost-recovery model would be a good solution, assuming a private-sector organization would be willing to accept the full commercial risk associated with this model.
Government of Canada Response
In 2008, the Government of Canada accepted the decision of the Board of Directors of AECL to discontinue the Dedicated Isotope Facility project (also known as the MAPLEs).
The Government of Canada notes the Panel’s assessment that there are considerable challenges associated with the DIF/MAPLEs project, including economic, technical and regulatory challenges. The Government will not invest additional public funds into this project. In line with the Panel’s recommendation, the Government will remain open to considering private sector proposals that would cover full costs, liabilities and risks without further public investment.
Conclusion
Supplies of Tc-99m will remain fragile in the short term. Continued dependence on the world’s five aging, government-funded nuclear reactors for production of Tc-99m is unsustainable.
The Government of Canada has asked AECL to make return to service of the NRU its top priority, and the federal budget has provided money for AECL’s ongoing operations including relicensing activities. Moreover, the Government has developed an action plan to increase the security of medical isotope supply for Canadians in the longer term based on i) encouraging new long-term supply of medical isotopes by investing in research and development to prove new technologies with commercial potential, ii) supporting resiliency and effective health system management by optimizing the use of available supplies and alternatives, where appropriate, and iii) continuing to work with the international community to coordinate outages and production of medical isotopes.
More specifically, and in response to the Panel’s recommendations, the Government of Canada will encourage new long-term supply of medical isotopes by implementing a number of concrete measures.
The Government will invest $35 million in research, development and demonstration to encourage the commercialization of non-reactor-based technologies for the production of Tc-99m. The program will advance technologies at the leading edge of isotope technology development and will help Canada remain a leader in the area of isotope technology. If successful, these technologies would deliver innovative solutions on a scale commensurate with Canada’s needs and would result in reduced nuclear waste.
Also, the Government of Canada will support increased efficiency in the use of Tc-99m and diversification of imaging modalities, in order to promote resiliency and enable effective health system management. Targeted investments will include $3 million for the development of tools, protocols and standards and $10 million to create a clinical trials network to help move research on isotopes into clinical practice.
The Panel has provided a very insightful and comprehensive report, which has guided the Government’s consideration of all available options. The Government of Canada wishes to thank the Panel for its hard work, and believes that with the implementation of these measures, Canadian supply of medical isotopes in the long term will be commercially viable, more secure, and more reliable.
Page details
- Date modified: